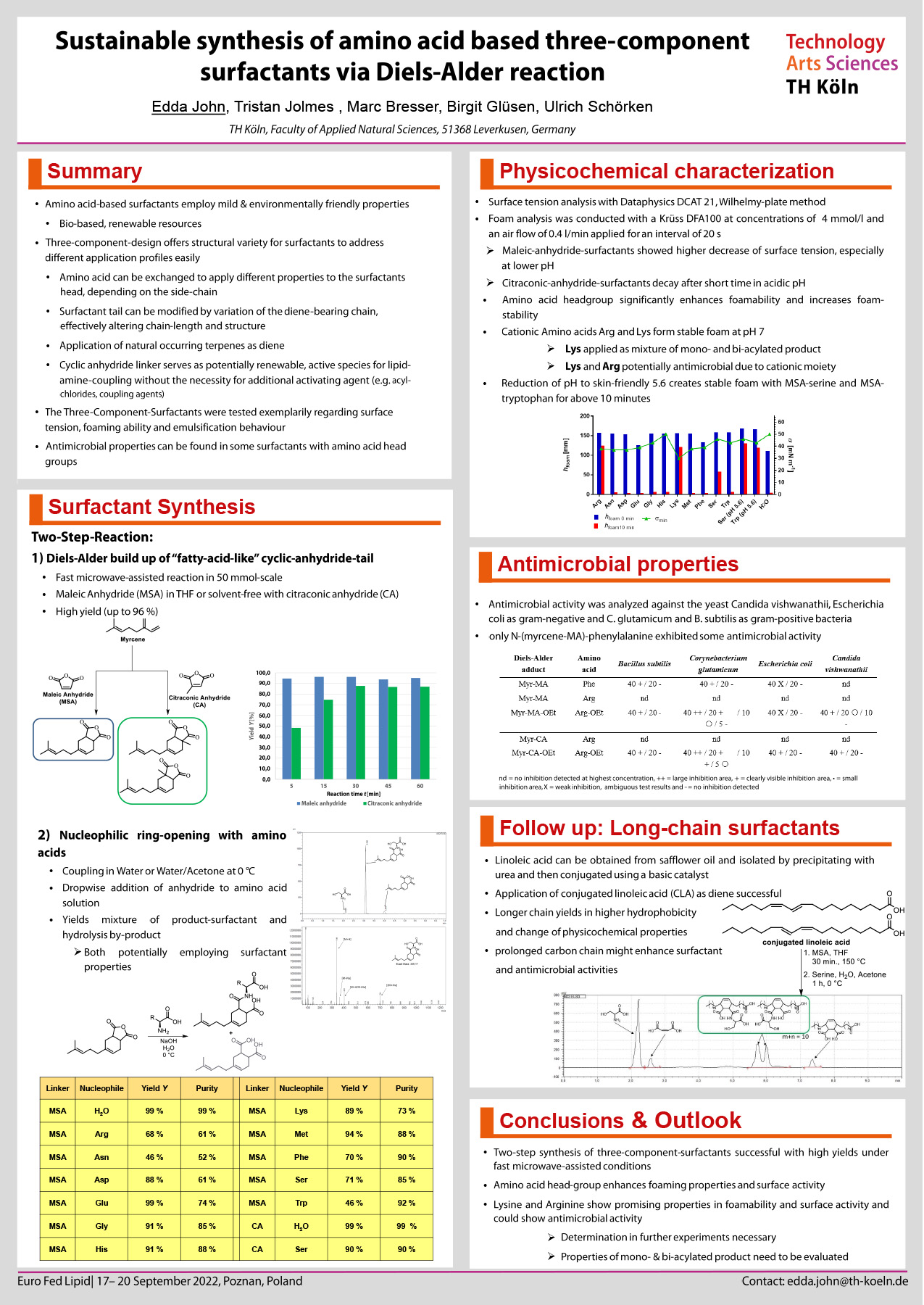
Surfactants play an indispensable role in many areas of industrial processes including detergents, food and pharmaceutical additives or cosmetic ingredients [1]. As the demand for sustainability continues to grow, the development of innovative and sustainable surfactants is of vital importance [2]. Amino acid-based surfactants are generally environmentally friendly, sustainable and have mild properties [3]. Therefore, we aimed at developing novel bio-based amino-acid surfactants starting from conjugated dienes, linker molecules, amino acids and optionally a short chain alcohol for carboxylic group esterification. In a two-step synthesis, the terpenoid myrcene was combined with the cyclic anhydrides maleic and citraconic anhydride in high yields via microwave-assisted Diels-Alder reaction. Subsequently, nucleophilic ring opening with amino acids was performed in an aqueous environment. Using this method, we were able to convert amino acids with good yields of up to 90%. In the case of citraconc anhydride as precursor, a near 100 % atom efficiency was achieved under solvent-free conditions, thus the synthesis strategy is in good accordance to the “12 Principles of Green Chemistry”. Initial physicochemical characterization showed best surface tension reduction and foam stability with amphoteric surfactants derived from arginine and lysine. Upon esterification with ethanol cationic surfactants were obtained, which exhibited a moderate antibacterial activity against Bacillus subtilis, Corynebacterium glutamicum, and Escherichia coli. Surface tension reduction of the amino acid surfactants with two or three carboxylic groups was quite low, indicating an imbalance in the size of the hydrophilic and hydrophobic parts. To overcome this limitation, longer chain dienes may be employed for the Diels-Alder cycloaddition to achieve larger hydrophobic tails. The synthesis of conjugated linoleic acid (CLA) and application as diene in the synthesis of more hydrophobic three-component surfactants is currently being investigated in our group.
[1] Nagtode VS, Cardoza C, Yasin HKA, Mali SN, Tambe SM, Roy P, Singh K, Goel A, Amin PD, Thorat BR, Cruz JN, Pratap AP., ACS Omega 2023, 8, 11674-11699.
[2] Le Guenic, S., Chaveriat, L., Lequart, V., Joly, N., Martin, P., J Surfactants Deterg, 2019, 22: 5-21.
[3] Ananthapadmanabhan, K.P., Tenside Surf Deterg 2019, 56, 378-386.