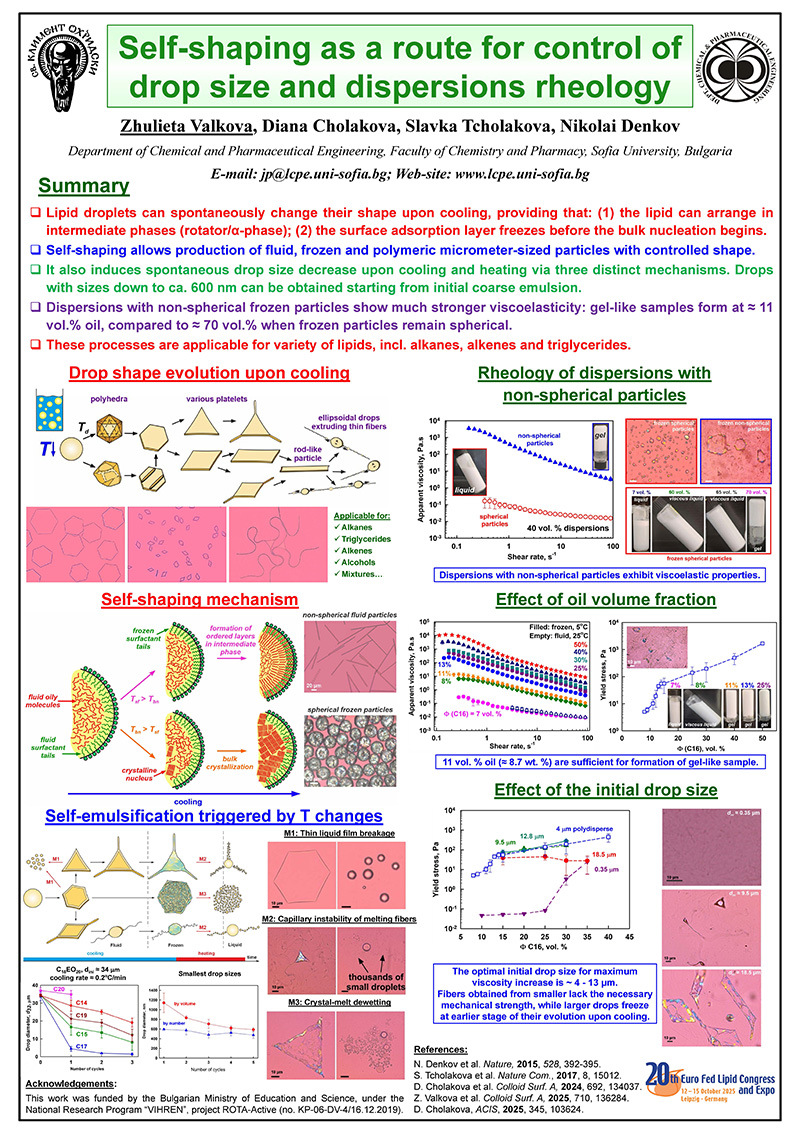
Lipid droplets can spontaneously change their shape upon cooling, providing that: (1) the lipid can arrange in intermediate phases (rotator/α-phase); (2) the surface adsorption layer freezes before the bulk nucleation begins.
Self-shaping allows production of fluid, frozen and polymeric micrometer-sized particles with controlled shape.
It also induces spontaneous drop size decrease upon cooling and heating via three distinct mechanisms. Drops with sizes down to ca. 600 nm can be obtained starting from initial coarse emulsion.
Dispersions with non-spherical frozen particles show much stronger viscoelasticity: gel-like samples form at ≈ 11 vol.% oil, compared to ≈ 70 vol.% when frozen particles remain spherical.
These processes are applicable for variety of lipids, incl. alkanes, alkenes and triglycerides.