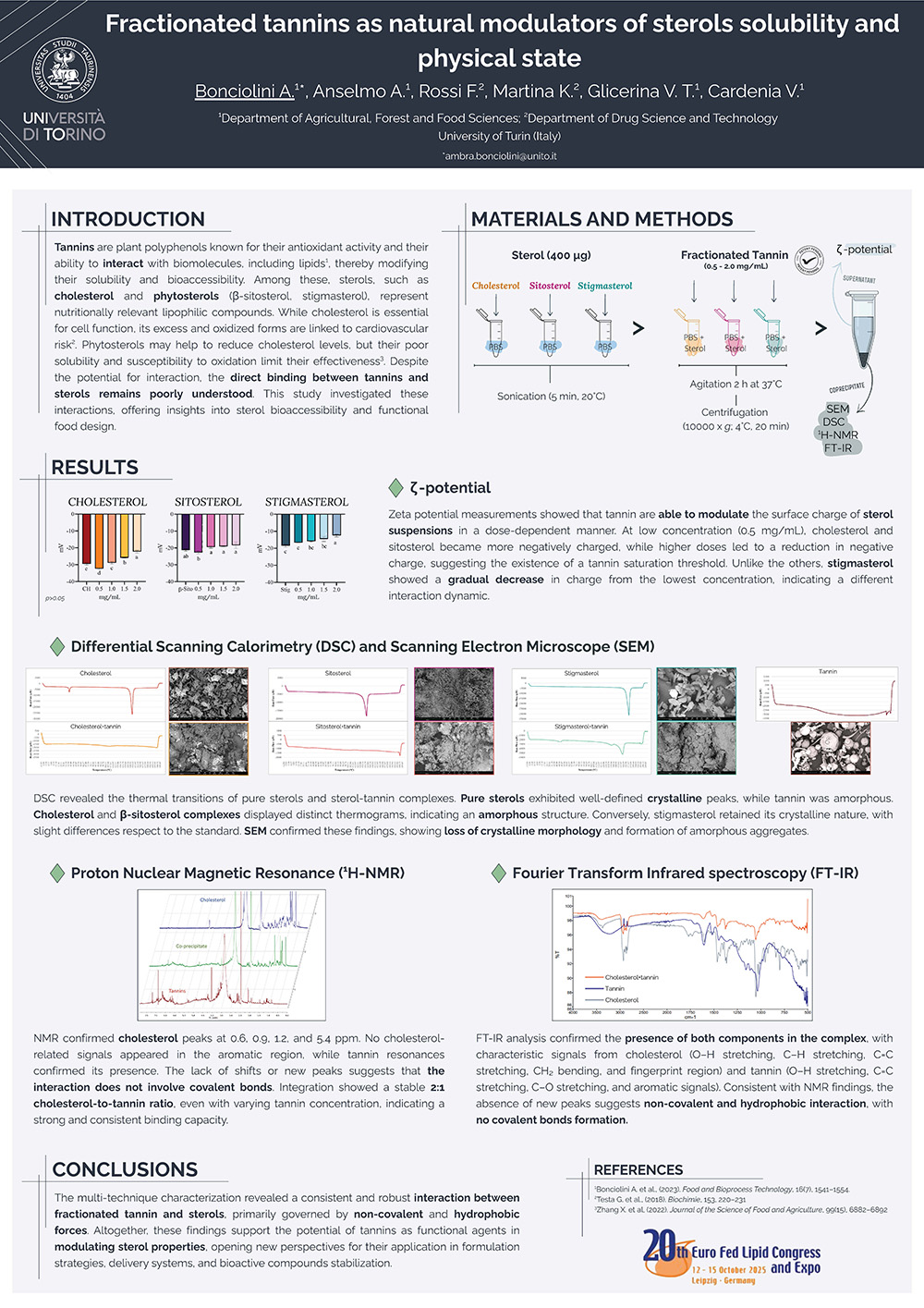
Tannins are polyphenolic compounds capable of interacting with biomolecules and altering their functional properties, including solubility. This study explored the interaction between a fractionated tannin extract (T, 0.1 mg/mL) and three sterols (cholesterol (CH); β-sitosterol (Si); and stigmasterol (St)) with a focus on co-precipitate formation, interfacial charge, and structural behavior.
Zeta potential analysis showed that low concentrations of tannin significantly (p<0.01) increased the negative surface charge of CH (−32.29 ± 0.37 mV) and Si (−22.69 ± 0.83 mV) compared to the sterols alone (CH: −29.46 ± 0.23 mV; Si: −21.28 ± 0.70 mV; p<0.01), suggesting improved dispersion in aqueous environments. Scanning Electron Microscopy (SEM) revealed amorphous co-precipitates, morphologically distinct from both pure tannin and sterols. Differential Scanning Calorimetry (DSC) confirmed a loss of crystallinity in CH and Si, as their characteristic melting peaks disappeared following tannin addition. In contrast, St retained its endothermic peak, though shifted from 172.3°C to 160.6°C with a marked reduction in transition enthalpy (−30971 μW to −5243 μW). 1H-NMR in DMSO-d6 indicated non-covalent interactions between CH and tannin, with a stable ~2:1 CH:T ratio, and no evidence of covalent bond formation. FT-IR spectra supported these non-covalent interactions.
These results demonstrated that tested fractionated tannin can form stable, non-covalent complexes with sterols, promoting their dispersion and reducing crystallinity. That interaction offers promising potential for the design of functional food and nutraceutical formulations where sterol solubility and bioavailability are critical.