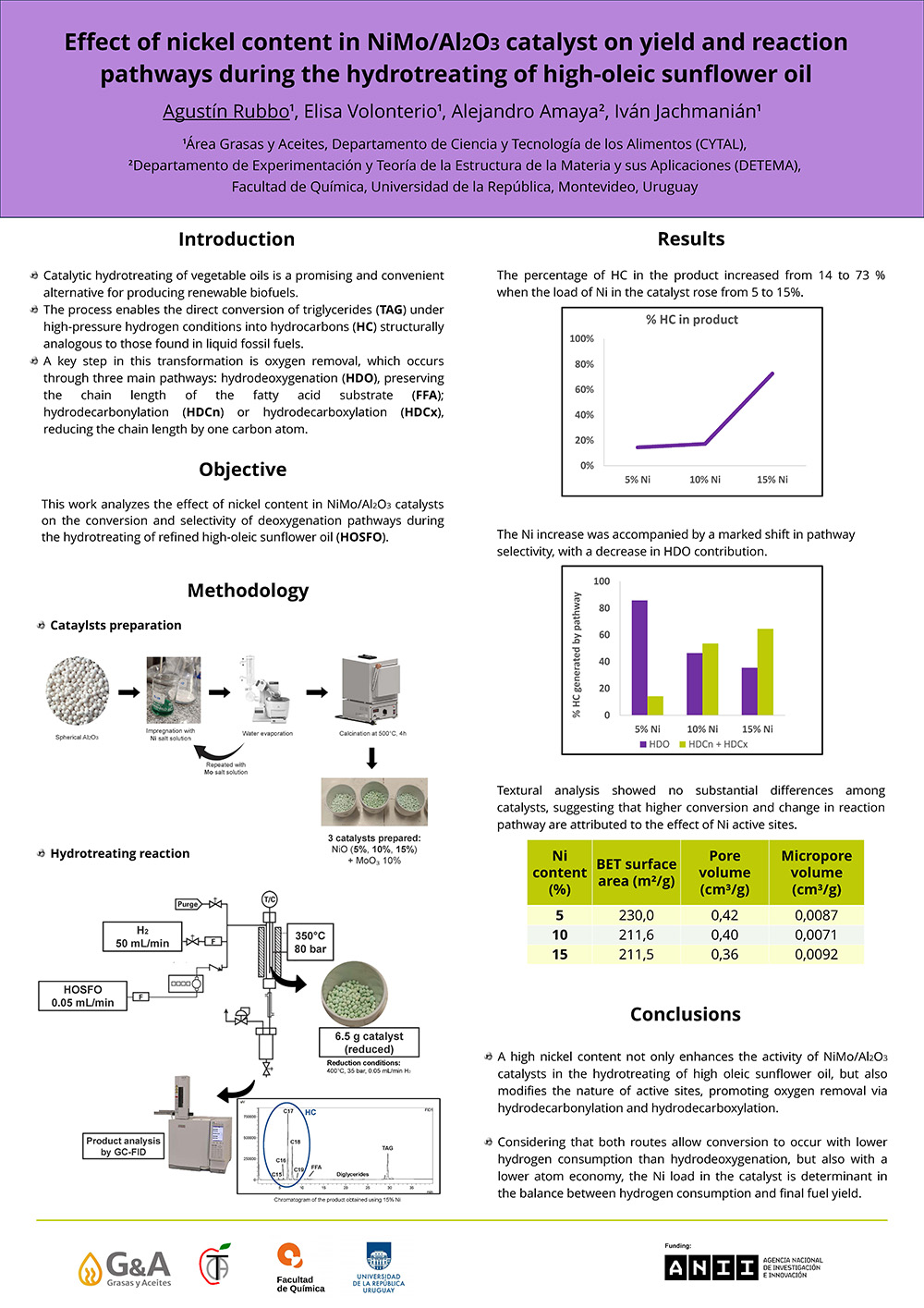
Catalytic hydrotreating of vegetable oils is a promising and convenient alternative for producing renewable biofuels. The process enables the direct conversion of triglycerides (TAG) under high-pressure hydrogen conditions into hydrocarbons (HC) structurally analogous to those found in liquid fossil fuels. Bimetallic systems based on transition metals, such as nickel and molybdenum, supported on alumina (NiMo/Al2O3), have demonstrated high efficiency in catalyzing this process. A key step in this transformation is oxygen removal, which occurs through three main pathways: hydrodeoxygenation (HDO), preserving the chain length of the fatty acid substrate; and hydrodecarbonylation (HDCn) or hydrodecarboxylation (HDCx), reducing the chain length by one carbon atom by eliminating carbonyl and carboxyl groups, respectively.
This work analyzes the effect of nickel content in NiMo/Al2O3 catalysts on the conversion and selectivity of deoxygenation pathways during the hydrotreating of refined high-oleic sunflower oil. Catalysts containing 5, 10 and 15% wt/wt Ni and 10% wt/wt Mo were prepared by incipient wetness impregnation on spherical Al2O3. The catalyst (6.5 g) was loaded in a tubular fixed-bed reactor and reduced under a H2 flow of 50 mL/min, at 35 bar and 400°C for 150 min. After catalyst reduction, pressure and temperature were adjusted to 80 bar and 350 ºC, respectively, and the reaction was initiated by feeding oil at a rate of 0.05 mL/min. After 3h of reaction, the product was collected and analyzed by GC-FID.
Results showed that the percentage of HC in the product increased from 14 to 73 % when the load of Ni in the catalyst rose from 5 to 15%. Furthermore, this increase was accompanied by a marked shift in pathway selectivity, while the percentage of HC generated by the HDO route decreased from 86 to 35 %, respectively. These results suggest that a high nickel content not only enhances the activity of the bimetallic catalyst but also modifies the proportion and nature of active sites, promoting oxygen removal via HDCn and HDCx. Considering that both routes allow the conversion to occur with lower hydrogen consumption than HDO, but also with a lower atom economy, the Ni load in the catalyst is determinant in the balance between hydrogen consumption and final fuel yield.